Abstract
Background: Melphalan exposure as measured by area under the curve (AUC) has a 5-fold variability between patients, with higher AUCs associated with improved outcomes but increased toxicity (Shaw BBMT 2012). Propylene glycol free melphalan (Evomela®, PGF-MEL) is an intravenous formulation with improved stability and possibly less toxicity related to the solubilizing agent. We have switched to PGF-MEL as conditioning prior to AHCT for multiple myeloma (MM) and AL amyloidosis (AL) and aimed to determine the pharmacokinetics (PK) and relationship between exposure and toxicities with this formulation in order to optimize dosing and personalize care in the future.
Methods: Serum samples were prospectively drawn at 5, 15, 30, 40, 75, and 150 min after administration of PGF-MEL given as a single dose on day -2 prior to AHCT. We calculated the MEL concentration in each sample using liquid chromatography tandem mass spectrometry, and AUC PK modeling was done with Phoenix WinNonlin v6.4. Toxicities were retrospectively collected using CTCAE v4 through day +100 after AHCT and compared by using either a T-test or Fisher's exact test between patients above and below the median AUC, 1st MEL level, and 6th MEL level.
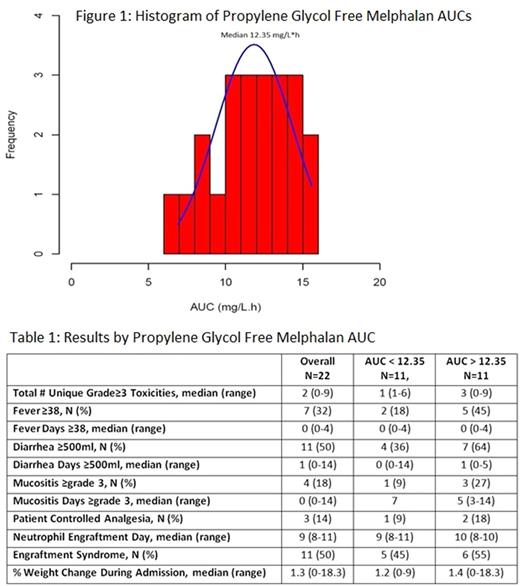
Results: Twenty-two patients (19 MM, 3 AL) with 68% male, median age 57yr (range 40-75), median creatinine 0.85mg/dL (range 0.6-2.9), median albumin 3.9g/dL (range 2.4-4.6), median weight 85.7kg (range 55-114kg), median body surface area 2.03 (range 1.6-2.3), received PGF-Mel at 140 (14%) or 200mg/m2 (86%). Median PGF-MEL AUC, 1st MEL level, and 6th MEL level were 12.35mg/L*h (range 6.9-15.6, Figure 1), 9750 ng/mL (range 5645-25000), and 1045 ng/mL (546-1566), respectively. Baseline characteristics and lab values were not statistically significant between patients with AUC above and below the median. Neutrophil engraftment occurred at a median of 10 (range 8-10) vs 9 (range 8-11) days post AHCT and length of stay was 16 (range 14-30) vs 16 (13-19) days for patients with AUC above and below the median, respectively. The median total number of unique grade ≥3 toxicities up to day 100 was 3 (range 0-9) vs 1 (1-6). Cardiovascular, infectious, metabolic, oral/gastrointestinal (GI), pulmonary, renal, and skin toxicities were seen in 36 vs 45% (p=.99), 45 vs 27% (p=0.66), 45 vs 36% (p=0.99), 55 vs 55% (0.99), 0 vs 9% (0.99), 9 vs 0% (0.99), and 55 vs 9% (p=0.06), for patients with above and below the median AUC. More patients had fevers (45 vs 18%), ≥500ml of diarrhea per day (64 vs 36%), grade ≥3 mucositis (27 vs 9%), and need for patient controlled analgesia (18 vs 9%), but these were not statistically significantly different between the groups (Table 1). As a surrogate for fluid overload, we measured the % weight change at discharge from the AHCT admission. Most patients lost weight with a median weight change of 1.4% (range 0-18.3%) vs 1.2% (range 0-9%) in those above and below median AUC, respectively. Engraftment syndrome occurred in 50% of patients overall (55% above vs 45% below median AUC). Comparisons above and below median 1st and 6th MEL levels were also not statistically significant. No patients progressed or died during the 100 day follow-up.
Conclusion: In this study, PGF-MEL had less variability in AUC than previously published for PG-MEL. Baseline characteristics were not different between patients with higher or lower MEL exposure. Toxicities occurred more frequently in patients with higher than the median AUC, but these differences were not statistically significant, likely due to the small number of patients. Longer follow-up is needed to assess disease response and outcomes. Ongoing assessment may identify optimal dosing and allow for personalization of therapy and better quality of life during the AHCT process. Additional patients with longer follow-up will be presented at the meeting.
Landau: Spectrum Pharmaceuticals: Other: Advisory Board, Research Funding; Celgene: Other: Advisory Board; Karyopharm: Consultancy; Pfizer: Honoraria; Takeda: Research Funding; Amgen: Research Funding; Janssen: Honoraria. Koehne: Atara: Consultancy, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal